Abstract
Despite the excitement of targeted therapies such asibrutinib and idelalisib, the longer-term follow-up offered by early immuno/-chemotherapy trials presents opportunities to explore the disease features that contribute most to long-term outcomes.
We employed next generation sequencing (NGS) to identify somatic mutations targeting 22 genes in DNA from 499 LRF CLL4 cases, a trial with >12 years follow-up on all surviving cases. The analysis was performed using a bespoke TruSeq panel, with confirmation of key sub-clonal mutations using orthogonal approaches (Ion Torrent [n = 19], SureSeq [n = 27], and ddPCR [n = 30]). Germ-line DNA was unavailable, so only COSMIC annotated mutations were included in downstream analysis. Variant allele frequencies were adjusted for tumor purity using CD19+CD5+% to define the clonal cell fraction (CCF). Significant associations between gene mutations and a comprehensive panel of clinical and biological features reported in previous LRF CLL4 papers were identified by Fisher's Exact test. Univariate Kaplan-Meier (KM) and multivariate Cox regression analyses were used to assess the impact of gene mutation status on progression-free (PFS) and overall survival (OS).
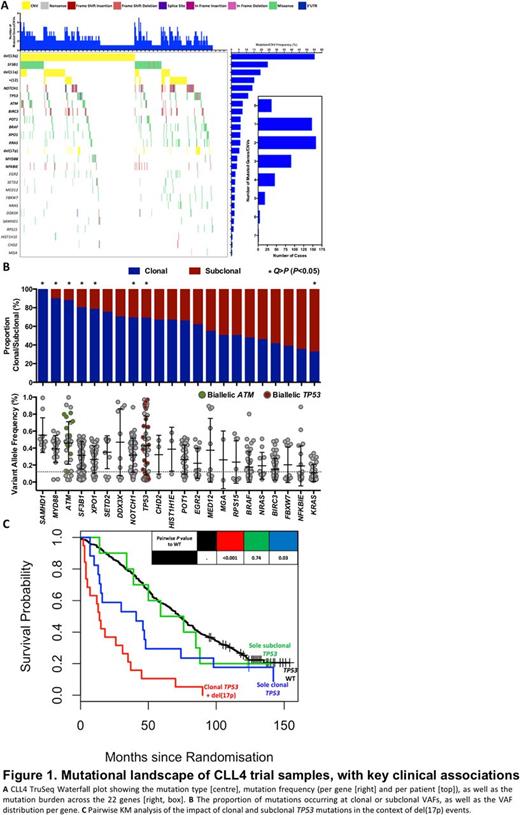
We identified 608 mutations (mean= 1.22, min/max = 0/7 per patient), with 93% of cases harbouring >=1 mutation or copy number alteration (CNA) (Fig1A). After correction for background mutation rate (0.5/Mb, >=3% recurrence), 11/22 genes were recurrently mutated at frequencies between 3% (NFKBIE) and 25% (SF3B1), (Fig1A). SAMHD1 and KRAS mutations were principally clonal and subclonal, respectively, while ATM, MYD88, NOTCH1, SF3B1, TP53, and XPO1 occurred at mostly clonal CCFs(two-way binomial test , False Discovery Rate [FDR], Q > P [ P <0.05]) (Fig1B). Co-occurrence analysis of these genes with available clinical/biological/genetic features (Fisher's Exact, n = 1799 tests), identified 31 associations (FDR, Q > P [ P <0.05]), including those with other mutated genes (e.g. SF3B1 and POT1, OR = 3.4, P <0.01), CNAs (e.g. TP53 and del(17p), OR = 111.2, P <0.001), biological features (e.g. NOTCH1 and prolymphocytes, OR = 3.7, P <0.001), and clinical outcome (e.g. KRAS and 10-year survival, OR<0.1, P <0.001).
Univariate survival analysis identified mutations of two genes associated with short PFS (TP53, EGR2) and nine with inferior OS (TP53, SF3B1, NOTCH1 +3'UTR , EGR2, RPS15, NFKBIE, BRAF, KRAS, NRAS). TP53 (median PFS = 7 months (mutated) vs. 26 (wild-type), HR = 1.95, P <0.001) and EGR2 (median = 13 vs. 25, HR = 2, P= 0.01) mutationswere associated with reduced PFS. In addition to the expected impact of TP53 (median OS = 31 vs. 73, HR = 2, P <0.001), NOTCH1 +3'UTR (median = 51 vs. 72, HR = 2, P= 0.001) and SF3B1 (median = 53 vs. 75, HR = 2, P <0.001) mutations, RPS15 (median = 34 vs. 70, HR = 2, P= 0.02) and NFKBIE (median= 43 vs. 70, HR = 2, P <0.01) also associated with reduced OS. Importantly, mutations of the MAPK-ERK genes, BRAF, KRAS, and NRAS, were principally mutually exclusive (87%), and all associated with reduced OS (BRAF : median = 46.5 vs. 71, HR = 1.69, P <0.01, KRAS : median = 46 vs. 70, HR = 1.87, P <0.01, NRAS : median = 50.5 vs. 70, HR = 2.22, P= 0.01). Only mutations in MYD88 associated with superior OS (median = 121 vs. 68, HR = 0.41, P= 0.01), but not in an analysis of only IGHV-mutated cases (median = 105 vs. 103, HR = 0.57, P= 0.27). Multivariate Cox models identified TP53, SF3B1, NOTCH1 +3'UTR, EGR2, as well as NRAS mutations (HR = 4, P <0.01) to predict for reduced OS.
Using Ion Torrent to confirm subclonal TP53 mutations (all IARC annotated) , we assessed the clinical impact of TP53 clonality in the context of del(17p) (Fig1C).Contrary to previous studies, whilst clonal TP53 lesions, and subclonal mutations with concomitant 17p loss were associated with reduced PFS and OS, solely subclonal TP53 mutations did not impact on outcome (OS: median = 59 vs. 73, P=0.74, PFS: median = 34 vs. 26, P=0.94).
Through resequencing analysis of the LRF CLL4 clinical trial, we identified several novel associations between gene mutations and clinic-biological disease features. We confirm the impact of several gene mutations on PFS and OS, and show that MAPK-ERK pathway mutations were associated with reduced survival. Furthermore, we provide evidence that subclonal TP53 mutations without detectable 17p loss do not exhibit reduced outcome, at least after initial treatment with chemotherapy.
Steele: Gilead: Consultancy, Honoraria; Portola Pharmaceuticals: Consultancy, Honoraria, Research Funding. Schuh: Abbvie: Honoraria; Janssen: Honoraria; Roche: Honoraria; Novartis: Honoraria; Gilead: Consultancy; Janssen: Honoraria; Gilead: Honoraria, Research Funding; Abbvie: Honoraria; Novartis: Honoraria; Celgene: Honoraria; Roche: Honoraria; Celgene: Honoraria. Strefford: Roche: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal